As of September 10, 2024, the US Food and Drug Administration (FDA) requires that all mammogram reports sent to patients must include breast density, which should be described as either “not dense” or “dense.”
If your breast tissue is not dense, the report will say, “Breast tissue can be either dense or not dense.
Broader commitment to prevent, detect and treat breast cancer
“Today’s action represents the agency’s broader commitment to support innovation to prevent, detect and treat cancer,” said Hilary Marston, M.D., M.P.H., FDA’s Chief Medical Officer. “Since 1992, the FDA has worked to ensure patients have access to quality mammography. The impact of the Mammography Quality Standards Act on public health has been significant, including a steep decrease in the number of facilities that do not meet quality standards. This means that more women have access to consistent, quality mammography. We remain committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer.”
The FDA amendments, which are required to be implemented within 18 months, enhance the FDA’s oversight of mammography facilities, including in the key areas of enforcement and patient communication. While nearly all certified mammography facilities continue to meet quality standards, today’s updates, among other things, enhance the FDA’s ability to communicate directly, if needed, with patients and their health care providers in cases where a facility did not meet quality standards and is not adequately communicating with patients about its deficiencies. This is intended to help ensure important information that could affect decisions about patient care, such as the potential need for further evaluation or a repeat mammogram, is communicated as completely as possible.
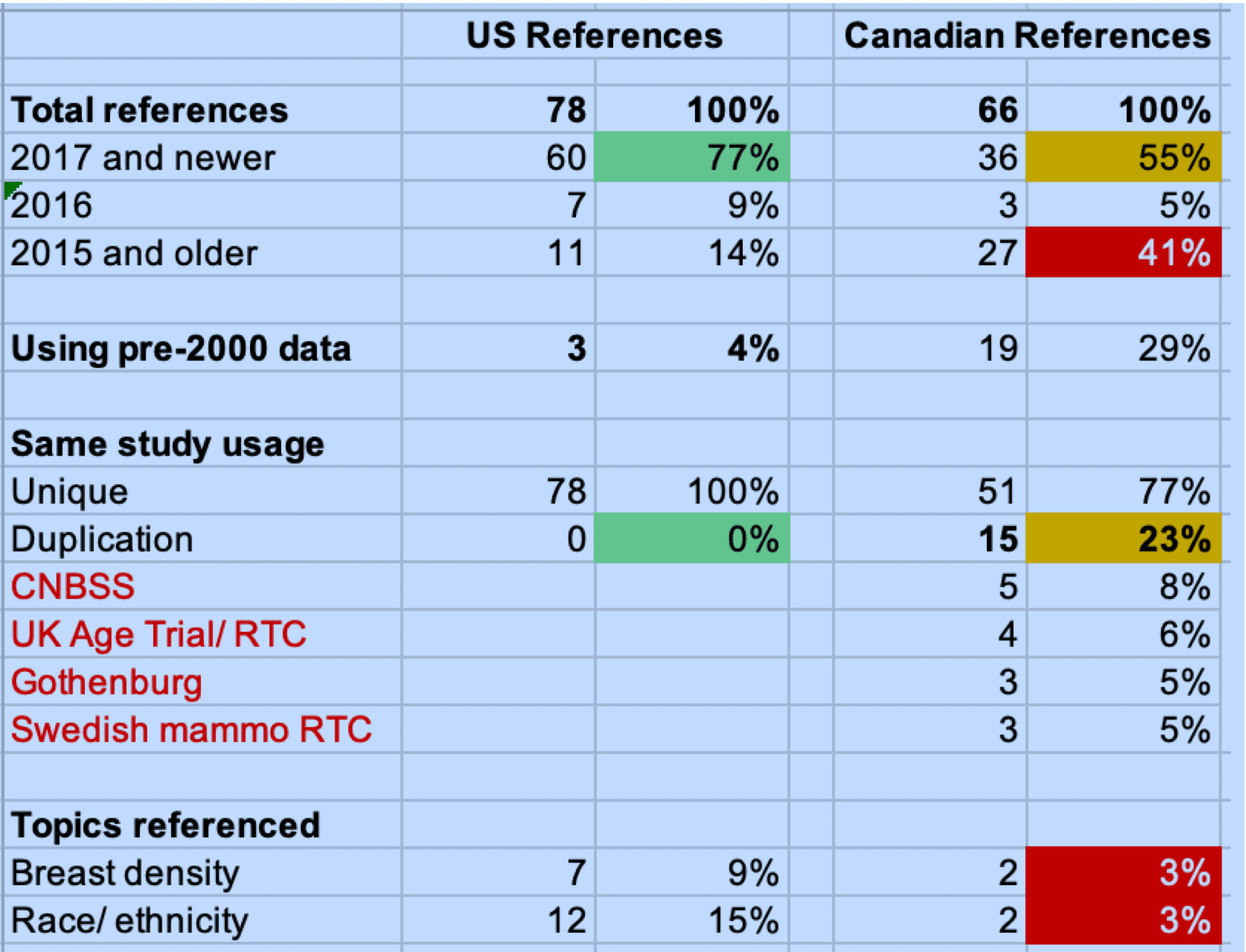
The announcement this month, and the news that women in the US will now be advised of their breast density comes as good news for organizations such as Dense Breasts-Info and Dense Breasts Canada who have battled over the last 10 years with governments in their respective countries to inform women of this critical piece of information. As I have written about on this blog, approximately 50% of the female population over the age of 40 have dense breasts. Dense breasts, which have very little fat, make it more difficult for a mammogram to detect breast cancer, as dense breast tissue presents as white (as does cancer) on imaging. Women with category C and D breasts should receive supplemental screening through an ultrasound. This, in my opinion, is where the new regulations fall short. It is welcome news that women will now be informed of their breast density, but do women know what to do with this information? Will they act on it? Do family doctors have clear clinical guidelines that require them to immediately order an ultrasound for their patients with category C and D density?
Dense breasts put you at higher risk
The communication to patients receiving a mammogram will say this:
If NOT DENSE: “Breast tissue can be either dense or not dense. Dense tissue makes it harder to find breast cancer on a mammogram and also raises the risk of developing breast cancer. Your breast tissue is not dense. Talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.”
If DENSE: “Breast tissue can be either dense or not dense. Dense tissue makes it harder to find breast cancer on a mammogram and also raises the risk of developing breast cancer. Your breast tissue is dense. In some people with dense tissue, other imaging tests in addition to a mammogram may help find cancers. Talk to your healthcare provider about breast density, risks for breast cancer, and your situation.”
This puts the onus on the woman to first, to read this report, which many do not fully. Second, they need to understand the implications of breast density. This explanation does an ok job of that. Third, there is a reference to other imaging, but nothing specific. Finally, it is suggested that women speak to their healthcare provider, which again assumes that they will take that step, and that the physician is open and receptive to that conversation.
Better education about breast density sooner
Not only that, but women need better education earlier about their breast density. It’s a message that simply is not sinking in. Is it because women don’t care? Is it because information isn’t being provided early enough? Is it that family doctors aren’t raising it as a topic for discussion? Or is it that women just don’t believe breast cancer will ever happen to them.
I recently spoke to an audience of 30 women and asked them point blank if they knew about breast density. Few were able to say they did. Most didn’t know what it meant. Even fellow breast cancer survivors in the room confessed they did not know of its implications for their health, and said they would be following up with their doctors to learn more.
So, I am both encouraged and discouraged by this month’s FDA’s announcement. Like most areas of women’s health, it is a step forward, but a baby step at best.