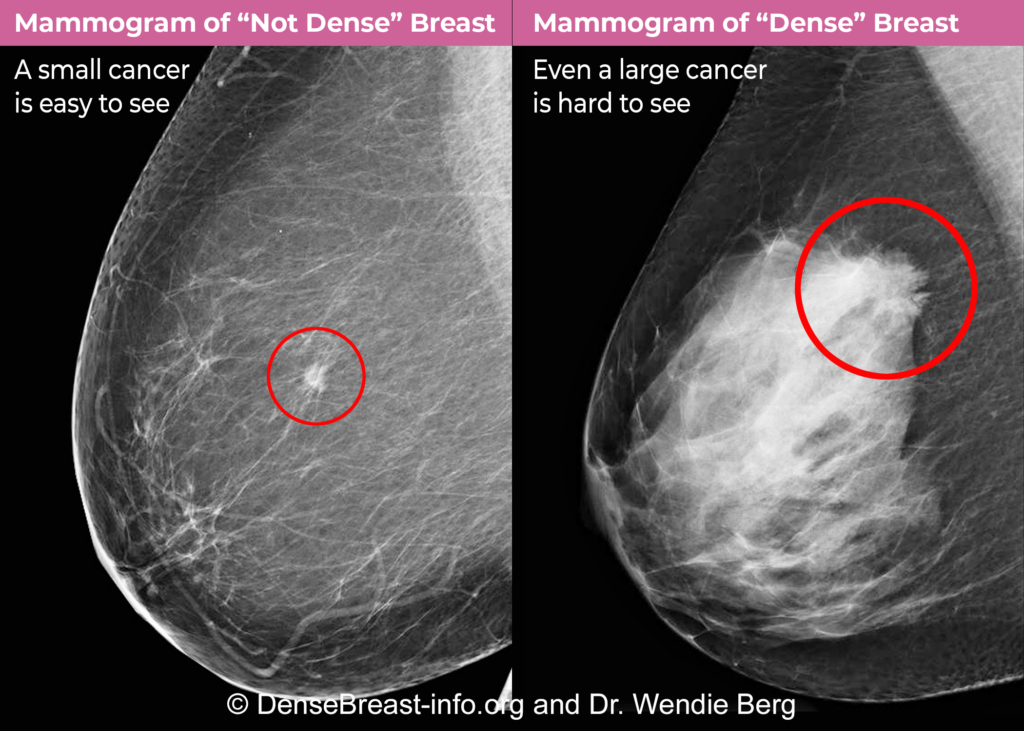
As of September 10, 2024, the US Food and Drug Administration (FDA) requires that all mammogram reports sent to patients must include breast density, which should be described as either “not dense” or “dense.” If your breast tissue is not dense, the report will say, “Breast tissue can be either dense or not dense. Broader …